Paper II of IV in my doctoral thesis:
Characterization of an Experimental Vaccine for Bovine Respiratory Syncytial Virus.
Sara Hägglund, Kefei Hu, Krister Blodörn, Boby Makabi-Panzu, Anne-Laure Gaillard, Karin Ellencrona, Didier Chevret, Lars Hellman, Karin Lövgren Bengtsson, Sabine Riffault, Geraldine Taylor, Jean-Francois Valarcher, Jean-François Eléouët
Clinical and vaccine Immunology: CVI 05/2014; 21(7). DOI:10.1128/CVI.00162-14
Abstract
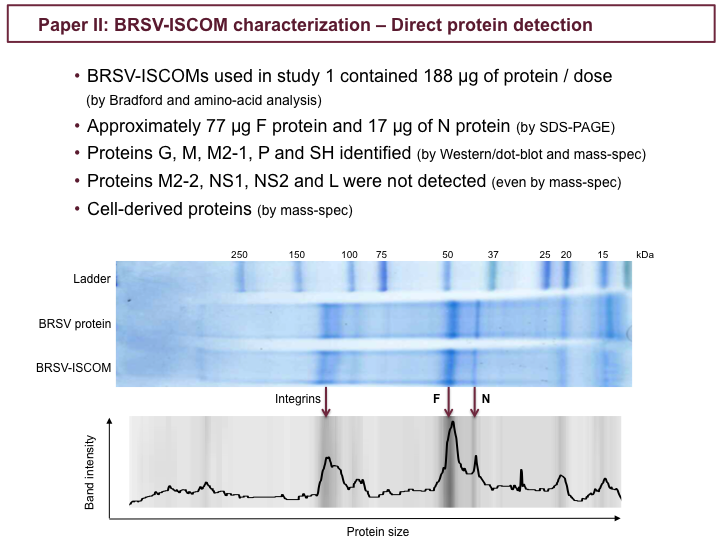
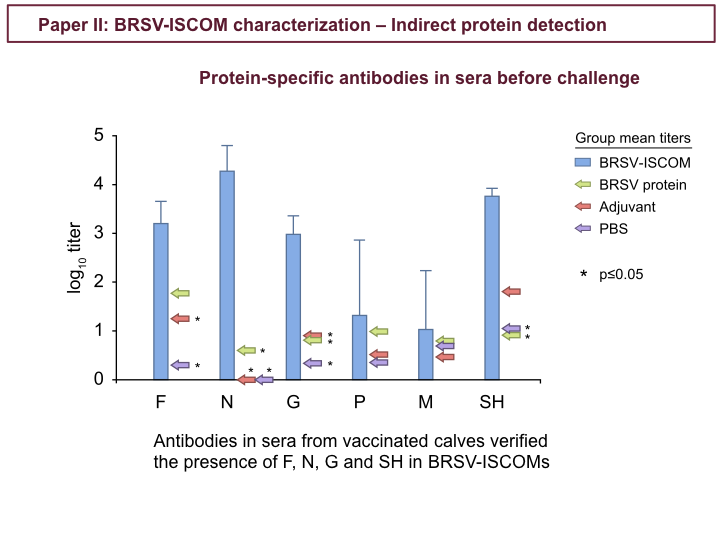
Bovine respiratory syncytial virus (BRSV) and human respiratory syncytial virus (HRSV) are major causes of respiratory disease in calves and children, respectively, and are priorities for vaccine development. We previously demonstrated that an experimental vaccine, BRSV-immunostimulating complex (ISCOM), is effective in calves with maternal antibodies. The present study focuses on the antigenic characterization of this vaccine for the design of new-generation subunit vaccines. The results of our study confirmed the presence of membrane glycoprotein (G), fusion glycoprotein (F), and nucleoprotein (N) proteins in the ISCOMs, and this knowledge was extended by the identification of matrix (M), M2-1, phosphoprotein (P), small hydrophobic protein (SH) and of cellular membrane proteins, such as the integrins αVβ1, αVβ3, and α3β1. The quantity of the major protein F was 4- to 5-fold greater than that of N (∼77 μg versus ∼17 μg/calf dose), whereas G, M, M2-1, P, and SH were likely present in smaller amounts. The polymerase (L), M2-2, nonstructural 1 (NS1), and NS2 proteins were not detected, suggesting that they are not essential for protection. Sera from the BRSV-ISCOM-immunized calves contained high titers of IgG antibody specific for F, G, N, and SH. Antibody responses against M and P were not detected; however, this does not exclude their role in protective T-cell responses. The absence of immunopathological effects of the cellular proteins, such as integrins, needs to be further confirmed, and their possible contribution to adjuvant functions requires elucidation. This work suggests that a combination of several surface and internal proteins should be included in subunit RSV vaccines and identifies absent proteins as potential candidates for differentiating infected from vaccinated animals.
Excerpt from my doctoral defense presentation
Classic BRSV-ISCOMs evaluated in study I induced exceptional protection in calves with BRSV-specific maternally derived antibodies (MDA). These BRSV-ISCOMs were produced using purified antigen from lysate of BRSV-infected cells and in this purification process only one fraction of the total antigen is used in the final vaccine. This leads to the question: What’s in the BRSV-ISCOMs?
In paper III, we refined and characterized a BRSV challenge model in calves and a set of tools to evaluate challenge outcomes, to facilitate further studies of vaccine candidate.